22 Which of the Following Has the Largest Ionic Radius
A metallic radius is one-half the distance between the nuclei of two adjacent atoms in a crystalline structure when joined to other atoms. Percentage values from the former region are reported as.
The feature of Class 10 Science Sample Paper for Term 2 CBSE Board Exam 2021-22 are the following.

. With a radius of about 10 15 meters a nucleus is quite small compared to the radius of the entire atom which is about 10 10 meters. This type of diagonal similarity is commonly referred to as diagonal relationship in the periodic table. Polymetallic nodules are also.
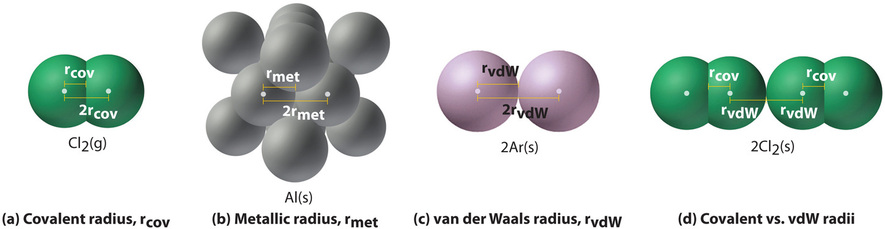
Versatile ionic gelatin-glycerol hydrogel for soft sensing applications. Covalent radius is the nominal radius of the atoms of an element when covalently bound to other atoms. All of the following properties of the alkaline earth metals increase going down the group except a atomic radius b first ionization energy c ionic radius d atomic mass e atomic volume 12.
And SectionC has 2 case based questions of 4. E Oxygen has a less negative electron affinity than fluorine. Determine the ionic strength mu for each of the following solutions.
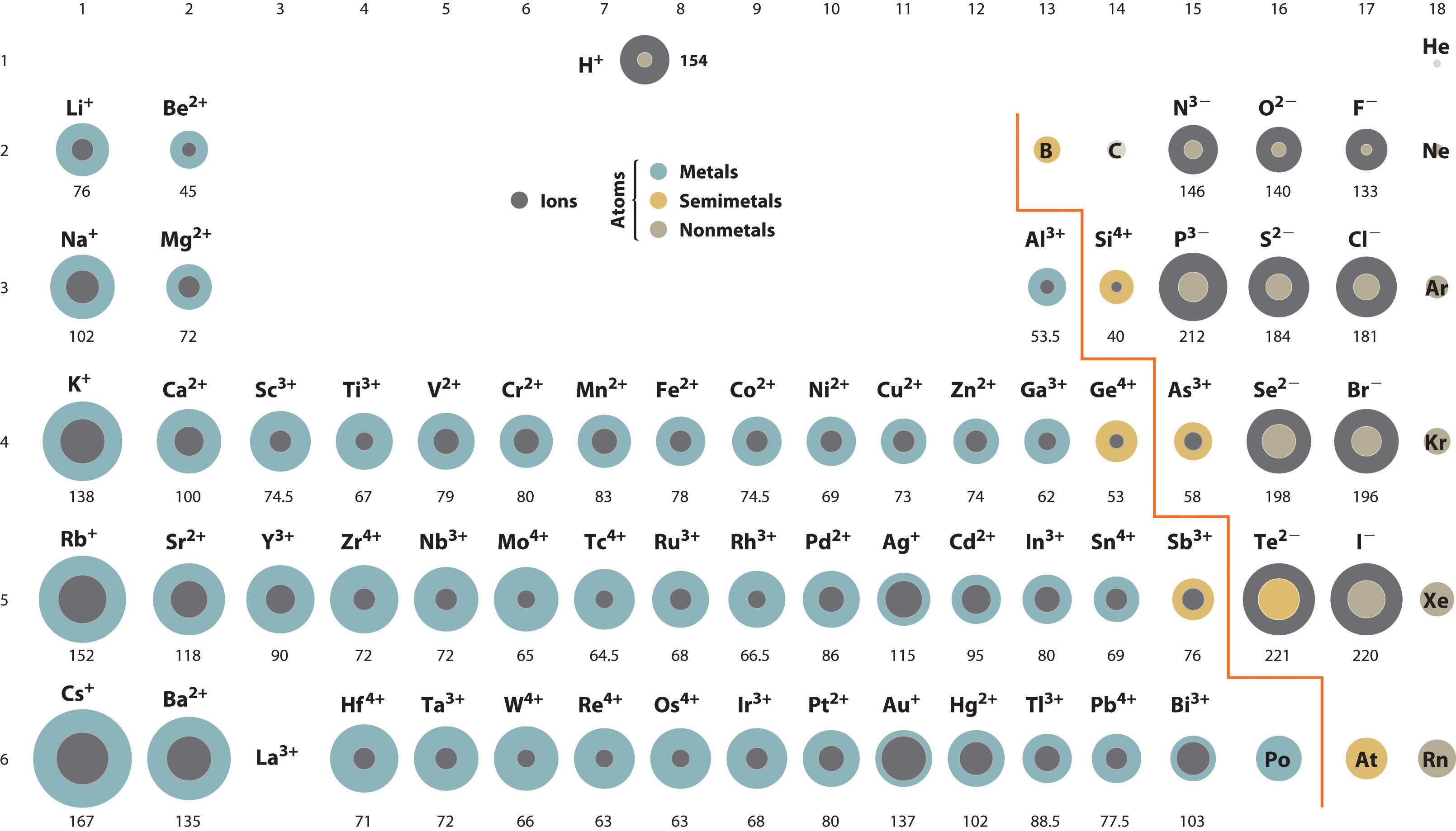
D The radius of a sodium atom is larger than that of a sodium cation. Their discovery of rubidium came the following year in Heidelberg Germany finding it in. Ii The question paper has three sections and 15 questions.
Hydrogen storage is a term used for any of several methods for storing hydrogen for later use. Thus lithium shows similarities to magnesium and beryllium to aluminium in many of their properties. Iii SectionA has 7 questions of 2 marks each.
Research has shown that deposits found just several 100 m apart can vary appreciably in compositionthe concentration of minerals in nodules found in the North Pacific belt is greater than the South Pacific. Distinct variations in the abs. These methods encompass mechanical approaches such as high pressures and low temperatures or chemical compounds that release H 2 upon demand.
A single protein type and for binary protein mixts. A-3 B29 C0 D38 E10 22 23Which one of the following cannot form a solid with a lattice based on the sodium chloride. IAll questions are compulsory.
It has the highest equivalent weight of any element and is the most unstable of the first 101 elements of the periodic system. All questions are compulsory. Fe 59 Halbach et al 1980.
No weighable quantity of the element has ever been prepared or isolated. For example water has a density of 1 gram per cubic centimeter and iridium one of the densest elements known has a density of 226 gcm. 22According to the phase diagram shown above the normal boiling point of this substance is _____ C.
The reason why ceNaF would have the highest melting point is because if you look at the electronegativity values fluorine has the highest hence it have the greatest pull or attraction towards the electrons in the ionic bond resulting in a really strong sodium positive ion and a strong negative fluorine ion this thereby results in a high melting point. The adsorption of lysozyme cytochrome c and myoglobin similar-sized globular proteins of 15 nm radius into the mesoporous silica material Santa Barbara Amorphous-15 SBA-15 with 33 nm mean pore radius has been studied photometrically for aq. A A solution of 000501 M NaOH mu b A solut.
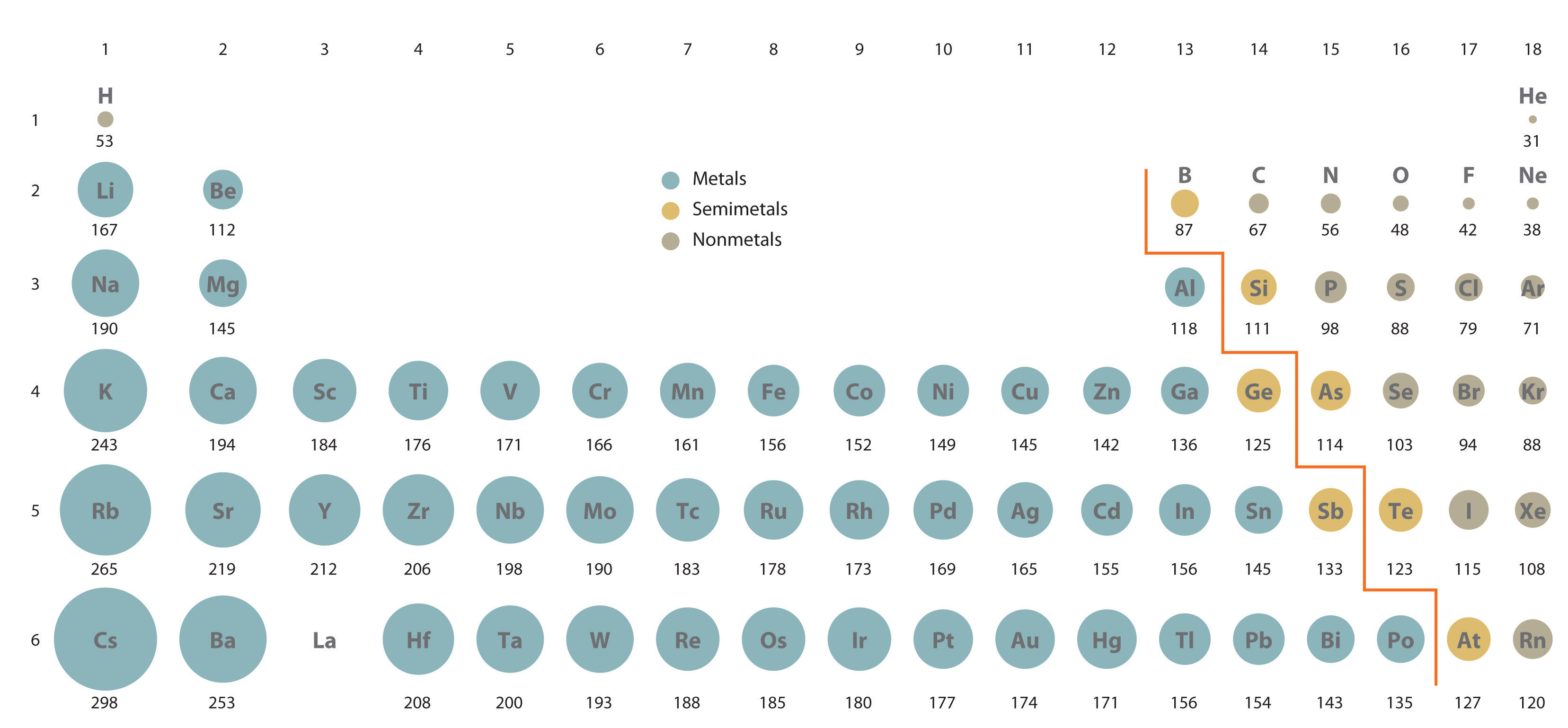
The sensing device is inexpensive and easy to manufacture is self-healable at room temperature can undergo strains of up. Due to having the lowest atomic weight and the largest atomic radius of all the elements in their periods the alkali metals are the least dense metals in the periodic table. As previously discussed when an atom has a larger atomic radius there is a larger shielding effect on its valence electrons.
The second element of the following group. The study of DNA electrophoresis began in 1964 when three groups of investigators 1-5 measured the mobility in free solution using moving boundary methodsThey found that the mobility was independent of size for DNA molecules larger than 400 base pairs bp and varied with ionic strength 3 5 and the identity and valence of the cation in the. While large amounts of hydrogen are produced it is mostly consumed at the site of production notably for the.
Lithium sodium and potassium are the only three. SectionB has 6 questions of 3 marks each. This is because of the increase in the number of core electrons due.
Assume complete dissociation of each salt and ignore any hydrolysis reactions. The diagonal relationship is due to the similarity in ionic sizes and or chargeradius ratio of the elements. To date the largest.
All known isotopes of francium are highly unstable so knowledge of the chemical properties of this element comes from radiochemical techniques. Nuclei are extremely dense compared to bulk matter averaging 18 10 14 grams per cubic centimeter. Alkali metals increase down the table with an exception at potassium.
An ionic radius is one-half the distance between the nuclei of two ions in an ionic bond.

Atomic Ionic Radius 1 1 4 Cie A Level Chemistry Revision Notes 2022 Save My Exams

Inorganic Chemistry Gap In Ionic Radii Chemistry Stack Exchange

Ionic Bonds Ionic Bonding Chemical Bond Ionic

Which Ion Has The Largest Radius

Periodic Trends In Ionic Radii Chemistry Libretexts Ionic Radius Ionization Energy Element Chemistry

Ionic Radius Definition Trends In Periodic Table With Videos

Cbse Class 11 Ionic Radius And Isoelectronic Species Offered By Unacademy

Atomic Ionic Radius 1 1 4 Cie A Level Chemistry Revision Notes 2022 Save My Exams
What Is An Ionic Radius What Is The Difference Between Atomic And Ionic Radius Quora
8 2 Atomic And Ionic Radius Chemistry Libretexts
Why Does Potassium Have A Larger Atomic Radius Than Sodium And Lithium Quora

Ionic Radius Ln 3 Vs Atomic Number Z Download Scientific Diagram

The Periodic Table Of The Elements Trends In Atomic Radius Electronegativity Ionizati Ionization Energy Periodic Table Of The Elements Chemistry Worksheets

Plot Showing The Ionic Radius Of Various Trace Element Ions In Download Scientific Diagram

Science Education Trends In Atomic Radius In The Periodic Table Teaching Chemistry Chemistry Classroom Chemistry Lessons

Atomic Ionic Radius 1 1 4 Cie A Level Chemistry Revision Notes 2022 Save My Exams

Comments
Post a Comment